The processing of brine electrolysis to produce caustic soda, chlorine, and hydrogen is called the electrolytic (chloralkali) process
Production process of caustic soda and chlorine by electrolysis
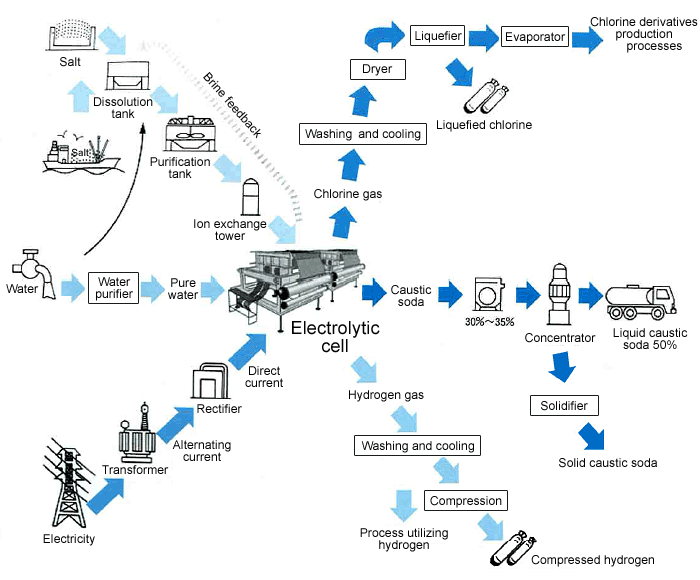 The processing of brine electrolysis to produce caustic soda, chlorine, and hydrogen is called the electrolytic (chloralkali) process. This is further classified into the membrane, diaphragm, and mercury processes. As of October 1999, all Japanese chloralkali plants use the membrane process.
The processing of brine electrolysis to produce caustic soda, chlorine, and hydrogen is called the electrolytic (chloralkali) process. This is further classified into the membrane, diaphragm, and mercury processes. As of October 1999, all Japanese chloralkali plants use the membrane process.
Salt is first dissolved in the dissolution tank. The obtained saturated brine is then sent to a purification tank to remove impurities, and to a chelate resin tower for purification before being fed to an electrolytic cell. Industrial water is also purified before entering the cell.
The anode chamber of the electrolytic cell is filled with the brine, and the cathode chamber with pure water (dilute caustic soda). Application of direct current to the cell produces chlorine gas at the anode, and caustic soda plus hydrogen at the cathode. The latter goes to the separator to produce a caustic soda solution with a concentration of about 30%.
The chlorine is washed and cooled to remove salt, and further dehydrated before being delivered as is, or liquefied.
Caustic soda is further concentrated in a vaporizer to a concentration of about 50% for delivery. Hydrogen is washed and cooled, as chlorine, before being shipped.
Principle of the chloralkali process
The electrolytic processes of brine to produce caustic soda, chlorine, and hydrogen may be categorized into three types: the membrane, diaphragm, and mercury processes. Here, the membrane process is described, since it is the only process presently operated in Japan.
This technology uses an ion-exchanging membrane separating the anode and the cathode. The membrane is made of a special resin which permits anions (negative ions) to pass through but not cations (positive ions).
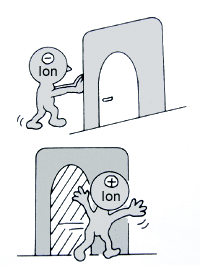
As shown in the figure, the anode chamber of a membrane electrolytic cell is filled with brine, and the cathode chamber with water (see figure). Application of an electric current leads to the formation of chlorine, caustic soda, and hydrogen.
The brine in the anode chamber contains sodium (Na+) and chloride (Cl-) ions. These ions migrate when a current is applied: the positively charged sodium ions pass through the membrane to the cathode chamber, while the negatively charged chloride ions are discharged on the anode surface to form chlorine gas (Cl2).
Water in the cathode chamber partly dissociates into hydrogen (H+) and hydroxide (OH-) ions. The hydrogen ions capture electrons on the cathode surface to form hydrogen gas (H2). The hydroxide ions are attracted to the anode, but blocked by the membrane, and react with the sodium ions from the anode chamber to form caustic soda (sodium hydroxide, NaOH).
(source: Japan Soda Industry Association)